| HTA Division Board Members
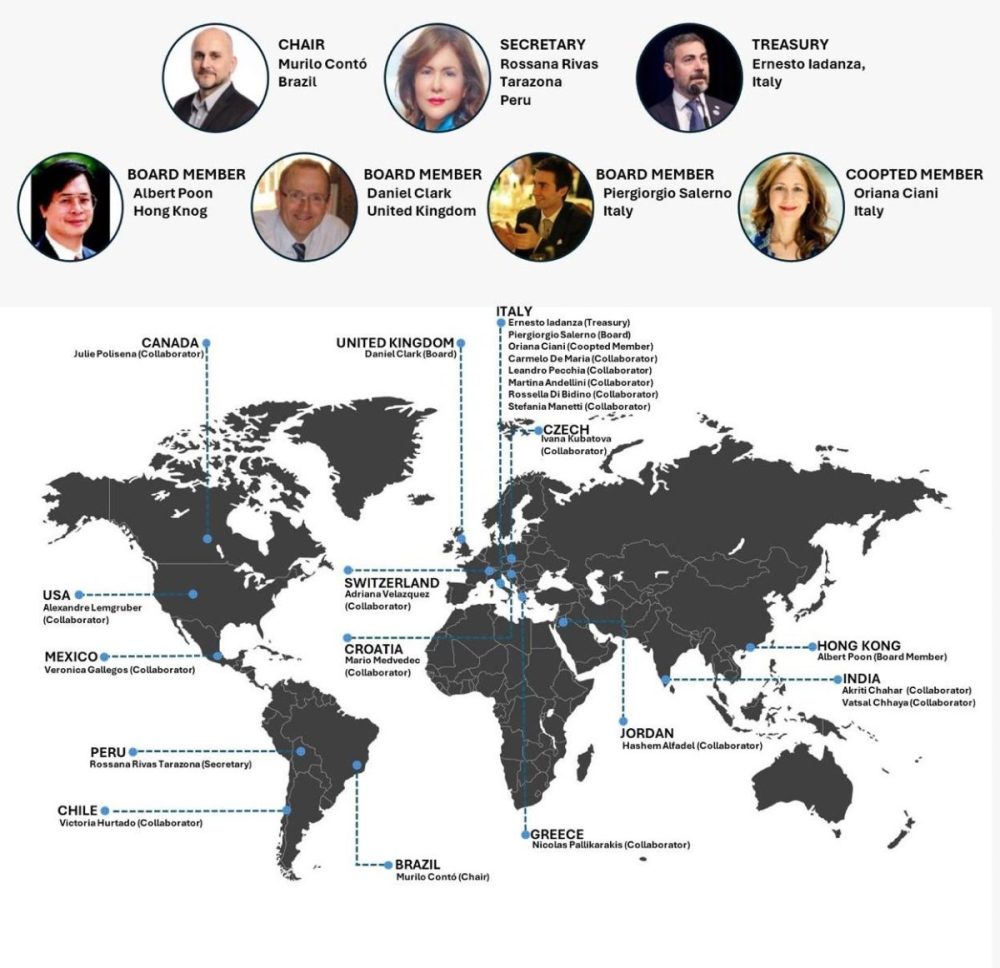
Chair

Murilo CONTÓ, Brazil
Chairman of the HTA Division
Specialist in Health Technology Assessment (HTA) and Health Technology Management (HTM) with more than 25 years working with medical devices, including activities in the industry, hospitals, federal government and international organization.
View ProfileMembers

Rossana Rivas TARAZONA, Peru
Secretary
Rossana Rivas Tarazona is MSc Management of Social & Health Organizations at Jean Moulin-Lyon III University, in Lyon, France; PhD Candidate, Mines ParisTech, Ecole des Mines de Paris, in Paris, France; International Consultant in Biomedical Eng., Clinical Eng., Health Technology Planning & Management and Health Technology Assessment. During the last 15 years she’s been interacting with Ministries of Health and other organizations from LATAM and the Caribbean region as others: USAID, the Rockefeller Foundation, Embassy of France, Embassy of Canada, UNESCO, etc. […]
View Profile
Ernesto IADANZA, Italy
Treasurer
Ernesto Iadanza, BME, CE, M. Sc., Ph.D., Adjunct Professor in Clinical Engineering at the Department of Information Engineering, University of Florence. National qualification as Associate Professor in Bioengineering.
View Profile

Albert POON, Hong Kong
Member
Engineer committed to advancing healthcare through the development of medical devices and equipment to benefit patients and communities. Provides professional expertise and technical advisory support to international organizations such as WHO, IFMBE, and LSHTM. Focused on strengthening medical device and IVD regulations in Asia and Africa, and supporting manufacturers in product registration to ensure affordable access to safe and effective devices in Hong Kong.

Daniel CLARK, United Kingdom
Member
Dan leads the Clinical Engineering service in Nottingham, one of the largest in Europe and provides the full scope of equipment services including: device evaluation, commissioning, service and maintenance…
View Profile
Piergiorgio SALERNO, Italy
Member
Biomedical Engineer passionate about statistical analysis, computer science, and artificial intelligence in healthcare. Dedicated to integrating medical knowledge with computational skills to optimize healthcare processes and improve care delivery efficiency. Focused on translating complex data into meaningful insights to guide informed decision-making and foster innovation in health systems.
Co-opted members

Oriana CIANI, Italy
Co-opted Member
Oriana Ciani is Associate Professor of Practice, Public Management and Policy Group, Health Economics & HTA at SDA Bocconi School of Management.
Her research interests are centred around the use and impact of Health Technology Assessment (HTA) on decision-making in healthcare, on the methodological aspects of the evaluation of health technologies, particularly medical devices, and on the use of evidence synthesis techniques to inform policy decisions and health policies evaluation. […]
Collaborators

Adriana VELAZQUEZ, Switzerland
Collaborator
With 40 years of experience in Biomedical Engineering, Adriana Velazquez is the senior adviser and focal point on medical devices at the World Health Organization (WHO), where she has been working since 2008, and leading the work to improve access to safe, affordable, quality and appropriate medical devices.
View Profile
Akriti CHAHAR, India
Collaborator
Researcher with a keen interest in HTA, presently working in IQVIA in HEOR and have experience working in the National Health Systems Resource Centre, Ministry of Health & Family Welfare. She’s skilled in program management, program evaluation, Systematic Reviews, Meta-analysis and Health Economic evaluation which enabled her to be associated with the Healthcare Division, WHO Collaborating Center for Priority Medical Device & Health Technology Policy. She’s 6 years of experience in prioritizing and assessing the health technologies & health programs at National Health Systems Resource Centre, Ministry of Health & Family Welfare, Government of India and currently at IQVIA.

Carlo FEDERICI, Italy
Collaborator
Carlo Federici is a lab researcher of Health Economics and Health Technology Assessment (HTA) at the Center for Research on Health and Social Care Management (CERGAS) of SDA Bocconi School of Management. At SDA Bocconi Carlo teaches the Advanced Decision Modelling course at the Master of International Healthcare Management, Economics and Policy (MIHMEP) as well as several courses on HTA and economic evaluations of healthcare technologies. […]
View Profile
Carmelo DE MARIA, Italy
Collaborator
Assistant professor in Bioengineering at the Department of Information Engineering of the University of Pisa, and affiliated with the Research Center “E. Piaggio ”of the same University. He is a member of the secretariat of the African Biomedical Engineering Consortium, and founder of the FabLab Pisa. He carries out research in the additive manufacturing field for applications in Biomedical Engineering (over 60 publications), and is among the promoters of open source design of medical devices through the UBORA platform
View Profile
Giuseppe FICO, Spain
Collaborator
Giuseppe Fico is heading the Health strategic area of the Life Supporting Technology research group at Universidad Politécnica de Madrid (UPM). He holds a PhD in biomedical…
View Profile
Hashem ALFADEL, Jordan
Collaborator
Dr. Hashem Al-Fadel is a seasoned Quality Consultant and lead hospital surveyor with extensive global experience in healthcare and quality management. He has held executive roles with GE Healthcare, Project HOPE-USA, and King Faisal Specialist Hospital, and served over 20 years as a founding board member and vice chairman of Istiklal Hospital in Jordan. Holding a B.S. in Biomedical Engineering, MBA, and PhD in Healthcare Management, he is board-certified in Clinical Engineering, a certified ISQua EEA and Temos surveyor, and has published over 60 articles and book chapters, actively shaping international healthcare standards and accreditation.

Ivana KUBÁTOVÁ, Czech
Collaborator
Head of Regulatory Affairs Department in B. Braun Medical s.r.o. Regulatory Affairs Specialist, Person Responsible for Regulatory Compliance (PRRC), Quality Manager, IVDR, MDR, ISO 13485, ISO 9001, GMP.

Julie POLISENA, Canada
Collaborator
Julie Polisena is a Senior Epidemiologist in the Marketed Health Products Directorate at Health Canada. She has a Master of Science in Health Services Research from the University of Toronto and earned her doctorate in Epidemiology at the University of Ottawa. In addition to her doctoral fellowship in health technology assessment (HTA) at the Society for Medical Decision Making, she gained extensive experience in HTA at the Agostino Gemelli University Polyclinic in Rome, Italy and the Canadian Agency for Drugs and Technologies in Health.

Kalliroi STAVRIANOU, Greece
Collaborator
Stavrianou Kallirroi is a post-doc researcher at the Biomedical Technology Unit (BITU), Laboratory of Medical Physics, University of Patras, Greece. She received her Diploma in…
View Profile
Leandro PECCHIA, UK
Collaborator
Leandro Pecchia received the degree in Biomedical Engineering in 2005 and the PhD in Economy and Management of Healthcare Services and Organizations in 2009 from the University “Federico II” of Naples…
View Profile
Mario MEDVEDEC, Croatia
Collaborator
Mario Medvedec earned his bachelor’s (BSc) and master’s (MSc) degree from the University of Zagreb Faculty of Electrical Engineering and Computing, and a doctoral (PhD) degree from the University of Zagreb…
View Profile
Marjan HUMMEL, The Netherlands
Collaborator
Marjan Hummel is a senior scientist in competitive intelligence & early health technology assessment at Philips Research in Eindhoven, the Netherlands. With a Master’s degree in Technical Business Engineering and a Doctoral degree in…
View Profile
Martina ANDELLINI, Italy
Collaborator
Martina Andellini completed her Master Degree in Biomedical Engineering at University of Rome “La Sapienza” in 2014. From May 2014 to September 2015 She worked as a researcher in neuroimaging focusing on the application of functional MRI for the study of various human brain disorders at Bambino Gesù Children’s Hospital in Rome. From September 2015 she joined the Unit of Health Technology Assessment at Bambino Gesù Children’s Hospital where she is still working on hospital-based health technology assessment (HTA) of medical devices. […]
View Profile
Nicolas PALLIKARAKIS, Greece
Collaborator
Nicolas Pallikarakis is Professor of Medical Physics and Head of the Biomedical Technology Unit (BITU). He also holds various key posts such as Director of the Postgraduate Program on Biomedical Technology at…
View Profile
Rossana CASTALDO, United Kingdom
Collaborator
Rossana Castaldo is Research Associate at the School of Engineering, University of Warwick. She holds a PhD and MSc in Biomedical Engineering from University of Warwick. She is working on the developments of new guidelines for Medical Devices (MD) for Low- and Middle-Income Countries.

Rossella DI BIDINO, Italy
Collaborator
Rossella Di Bidino is a senior HTA Expert at Fondazione Policlinico Universitario Agostino Gemelli IRCCS in Rome (Italy). She participates in the European Network for Health Technology Assessment (EUnetHTA) and the International Network of Agencies for Health Technology Assessment activities (INAHTA). For more than 5 years, she was member of the Secretariat of P&R-HTA office of Italian Medicines Agency. She has a Master’s in International Healthcare Management (MIHMEP) from Bocconi University and a doctorate in Management of Healthcare Organisation by Catholic University of Rome. She was visiting scholar at CADTH. She is member of HTAi and the International Society of Pharmacoeconomics and Outcomes Research (ISPOR).
View Profile
Vatsal CHHAYA, India
Collaborator
Principal Medical Writer | Evidence-Based Healthcare & HTA Specialist | Senior Clinical Researcher | Scientific Storyteller | BAPS Volunteer

Veronica GALLEGOS, Mexico
Collaborator
Deputy Director of Health Technology Policy Analysis

Victoria HURTADO, Chile
Collaborator
Researcher at ETESA, Chilean Ministry of Health
